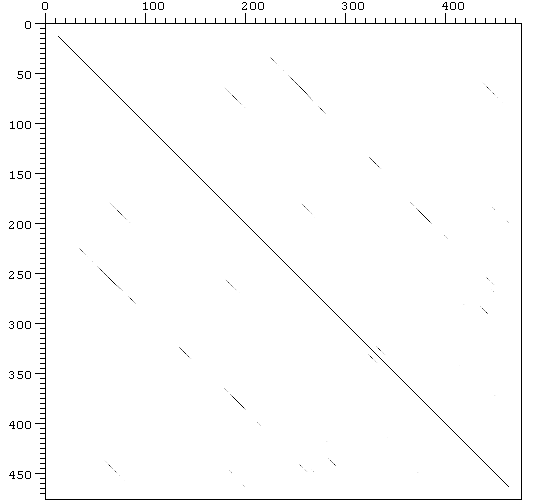
Figure 6. Human VTDB Dot plot
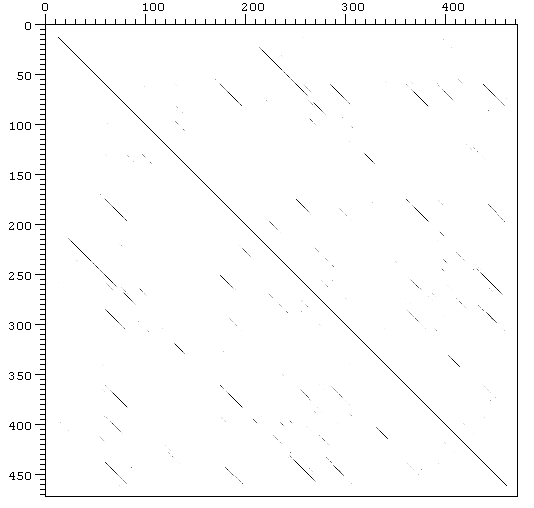
Figure 7. Mouse VTDB Dot plot
(Click to enlarge)
(Click to enlarge)
First of all, to study the serum albumin family, the protein sequences were obtained from a search of the nonredundant database maintained at the NCBI and by searches using the program BLASTP with the blast network server (Altschul et al. 1990).
With a e-value higher than 2.6 false positives started to appear.
The sequences used in this analysis are given in table 1 and are identified by accession number.
All sequences were initially
aligned with the program CLUSTALW
in order to identifie the conservative and the non conservative amino acid
replacement. Then, the sequences of the same protein were aligned.
Serum
albumin family
Vitamin D-binding
protein
Albumin
Afamin
Alpha-feto protein
As it can be seen in
the results, the proteins share structural similarities. Each protein
consists on three homologous domains of about 180 amino acids. Each domain
has a repeated structure consisting on 12 conserved cysteins that are cross-linked
by 6 internal disulfide bonds as shown in the following schematic representation:
+---+ +----+
+-----+
| | |
|
| |
'C': conserved cysteine involved in a disulfide bond.
'*': position of the pattern.
Figure 5. Conserved disulfide
bonds structure. (Swissprot)
This fact may indicate that the disulfide bonds determine the 3D structure of the protein and are the most important pattern in keeping the function of the proteins (act as carriers).
Moreover, there is a common pattern based on the three conserved cysteines at the end of the domain:
[FY]-x(6)-C-C-x(7)-C-[LFY]-x(6)-[LIVMFW]
This pattern can be
detected in the three domains, in each protein of the family (Swissprot).
The rest of the sequence
is poorly conserved, showing great divergence between the proteins. But,
when the different species sequences of the same protein were aligned,
the results show that each protein is highly conserved between vertebrates
(see Clustalw results).
Possibly, this is due
to the specialization in functions that suffered the different members
of the family throughout evolution; each protein has specialized in transporting
some metabolits (different between VTDB, ALB, AFP and AFM), and this function
is maintained in all the vertebrates.
The first domain is
conserved only in VTDB protein; in the rest of the family proteins, the
pattern of cysteines has been lost.
Protection of domain
I in VTDB during evolution may correlate with an important functional aspect
of its sequence (Yang et al. 1990).
The third domain in VTDB is shorter than the equivalent in the other proteins; this results from the deletion of two internal exons.
Of all four proteins,
VTDB is the most divergent. This can be related with the fact that VTDB
gene was the first gene in appear.
The three domain shown
in the clustalw seem to be homologous. This fact can be demonstrated using
Dotter
program which can compare each sequence with itself. The results are
shown in Fig. 6 (human VTDB) and Fig. 7 (mouse VTDB).


Figure 6. Human VTDB Dot plot
Figure 7. Mouse VTDB Dot plot
(Click to enlarge)
(Click to enlarge)
Two-dimensional plot showing periodically repeated similarities in the structure of VTDB. VTDB aminoacidic sequence was compared with itself, the major diagonals represent a perfect match of a sequence with itself and the minor diagonals represent the partial match between different regions of the same sequence.
The protein is known
to have a repetitive structure of three domains. This fact can be supported
with both of dot plots showed above. VTDB dot plot shows the similarity
between its 3 domains in both of the species, and this represent in fact
relics of early evolutionary events. These homologous regions can also
be observed in the structure of the rest of the members in VTDB family,
such as albumin or alpha-fetoprotein (Gibbs & Dugaiczyk,
1994).
Home | Introduction | Gene analysis | Protein analysis | Evolutionary study | References