

Selenium (Se) is an essential trace element and plays a key role on the homeostasis of many cellular and organismal functions (e.g. mammalian development, immune function and inhibition of viral infection). It is important in some pathophysiological conditions such as heart disease, neuromuscular disorders, cancer or inflammation.
Selenocysteine (Sec, U) is the 21st amino acid, and exists in all three domains of life. In fact, it is the major form of selenium in the cell. It was discovered by the North American scientist Thressa Stadtman at National Institutes of Health. This amino acid is present in several enzymes such as gluthathione peroxidases, thioredoxin reductases and some hydrogenases among others.
 Sec is encoded by in-frame UGA codons in the mRNA. Sec introduction in the polipeptidic growing chain requires two mechanisms:
Sec is encoded by in-frame UGA codons in the mRNA. Sec introduction in the polipeptidic growing chain requires two mechanisms:
- Trans-acting protein factor (Sec-tRNA [Ser] Sec)
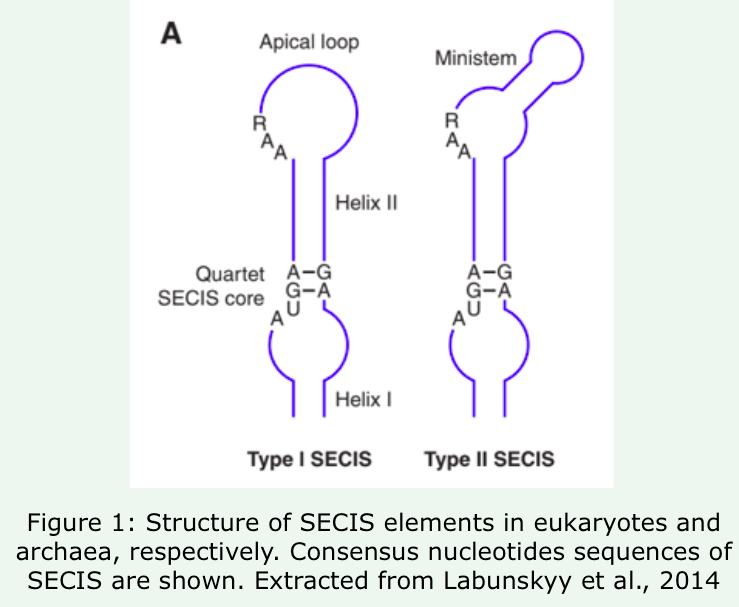
- Cis-acting Sec insertion sequence (SECIS). It is a stem-loop RNA structure found in 3'-UTR of all selenoprotein mRNA. As we see in the figure, there are 2 types of SECIS. RAA sequence is necessary for Sec incorporation, while Quartet SECIS score plays a major role in the interaction with a machinery protein named SBP2.
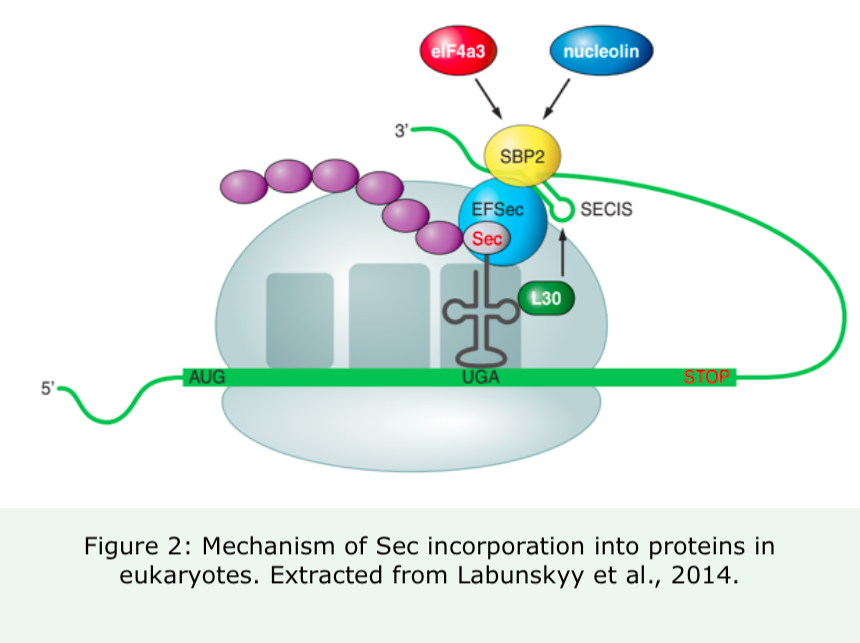
When a ribosome comes across an UGA triplet (which normally encodes for an STOP codon and promotes premature termination), Sec machinery interacts with translational machinery in order to prevent termination. SECIS dictates the re-coding of UGA into Sec, and Sec-tRNA [Ser] Sec, which has an anticodon complementary to UGA, translates UGA into Sec.
Other important proteins are also present during this process:
1-. SECIS binding protein (SBP2): it is indispensable for Sec translation. SBP2 is associated with the ribosome and interacts with eEFSec. It has 3 major domains: NH2 terminal (with unknown function), Sec incorporation domain (SID, which is a SECIS-binding enhancer) and COOH-terminal RNA-binding domain (RBD, which interacts with rRNA, snRNA and SECIS).
2-. Sec-specific translation elongation factor (eEFSec): it is also vital for Sec translation. Its main function is to recruit Sec-tRNA [Ser] Sec. The main function of eEFSec is to recruit tRNA [Ser] Sec and together with SBP2, inserts Sec in the protein sequence.
3-. L30
4-. eiF4a3 and nucleolin: play a regulatory role.
In molecular biology, any protein which includes a selenocysteine amino acid residue is considered a Selenoprotein. This kind of proteins exist in all domains of life: Eukaryotes, Bacteria and Archaea. Curiously, selenoproteins have been lost in Fungi, higher plants and in some insects (e.g. beetles and silkworms). Aquatic animals have really large selenoproteins, a fact which suggests that the environments play a pivotal role in the evolution of this special type of proteins. It has been demonstrated that selenoproteins are widely present in eukaryotes, expressed both in single-cell organisms and vertebrates, indicating their ancient origin. Recent studies have proved a decreasing trend in selenoprotein usage in mammals, but the cause is still unknown.
Normally Sec residue of selenoproteins is found in their active site to perform catalytic redox reactions.
Glutathione Peroxidases
GPx proteins are found in all 3 domains of life. They are involved in hydrogen peroxide signalling, detoxification of hydroperoxides, and maintaining cellular redox homeostasis. In mammals there are 8 GPx paralogs (GPx1, GPx2, GPx3, GPx4, GPx6). The other three homologous are GPx5 GPx7, GPx8 and the active-site Sec is replaced by Cys; thus they are not selenoproteins.
- GPx1 is the most abundant selenoprotein in mammals. It plays a role in the protection of cells from oxidative damage. It catalyzes glutathione (GSH)-dependent reduction of hydrogen peroxide to water, decreasing toxic H2O2 levels. Mammal GPx1 forms a homotetramer and is expressed in all cell types, but its highest expression is in the liver and kidney. As H2O2 is an important signaling molecule, both GPx1 deficiency and increased activity may affect H2O2-mediated responses.
- GPx2 is found in epithelium of the GI tract. It might have a dual role in cancer, by enhancing and preventing tumor growth.
- GPx3 is primary secreted from kidney and is the major GPx form in plasma.
- GPx4 is expressed in numerous cell types. It differs from GPx family due to its differential composition of the active site. Its main function is to reduce complex lipid hydroperoxides associated with membranes, thus prevents lipid decomposition. Moreover, this protein also prevents oxidative stress-induced apoptosis. Unlike the others GPx proteins, GPx4 also uses protein thiols as a donors of electrons, besides GSH. There are three different isoforms due to 3 different alternative splicing, that code for cytosolic, mitochondrial and nuclear GPx4 proteins. Cytosolic GPx4 is expressed ubiquitous both during embryonic development and in adults, but nuclear and mitochondrial forms are only expressed in testes.
- GPX6 is expressed only in olfactory epithelium during embryonic development. GPx6 homologs in some mammals are not selenoproteins, they have a Cys in their active site.
Thyroid hormone deodinase family
DI family is formed by three paralogous proteins in mammals: DI1, DI2 and DI3. Homologous proteins are found in other vertebrates and in simple eukaryotes, whose function is unknown. These proteins play a role in the regulation of thyroid hormone activity by reductive deodination. DI1 and DI3 are found mostly on the plasma membrane, whereas DI2 is localized to the endoplasmic reticulum (ER).
Deiodinases are integral membrane selenoproteins with a single transmembrane domain at the NH2-terminal region, where it is also found the active-site Sec residue. Normally, thyroid hormones are produced in its inactive form (T4), as prohormone. Once is secreted, it is converted to its active form (T3) by outer ring deiodination reaction, catalyzed by DI1 and DI2. This active form (T3) can be inactivated by DI3, leading the formation of rT3.
Thioredoxin Reductases (TXNRD)
TR are oxidoreductases that form the major disulfide reduction system of the cell, along with thioredoxin (Trx). There are three isoforms: TR1 is found in the cytosol and the nucleus; TR3 is located in the mitochondria which plays a role in the reduction of mitochondrial thioredoxin (Trx2) and glutaredoxin (Grx2); and TR2 that differs from the others in that it has an additional glutaredoxin domain.
TR1 is involved in the regulation of many transcription factors (such as p53), while it also maintains redox homeostasis and controls cell signaling.
Methionine-R-Sulfoxide Reductase 1
MsrB1 is a zinc-containing selenoprotein. It catalyzes the reduction of the R enantiomer of oxidized methionine residues in proteins. There is another enzyme which catalyzes the same reaction but for the S enantiomer, it is called MsrA. There are two homologs proteins which have Cys instead of Sec in their active site: MsrB2 that is localized in mitochondria and MsrB3 that is targeted to ER. As MsrB1 repairs oxidized methionines in proteins, it was proposed that this class of enzymes play a role in protective mechanism against oxidative damage to proteins.
Selenophosphate Synthetase 2
SPS2 catalyzes the synthesis of the active Se donor selenophosphate which is required for selenoprotein synthesis.
Rdx family
It is characterized by the presence of a conserved motif which is Cys-x-x-Sec and contains a thioredoxin-like fold. The followed proteins are contained in this family.
- SelenoW is found in the cytosol of muscular and brain cells. SelenoW forms a complex with glutathione. Its exact function remains unknown, but it has been proposed to have a role in redox regulation of 14-3-3 protein among others.
- SelenoT is localized to the ER and Golgi in both embryonic and adult tissues. By knocking down this gene, a wide range of SelenoT functions were identified. Among others, SelenoT is involved in cell structure organization, as well as in the regulation of several oxidoreductase genes. Further studies are needed to better understand SelenoT functions.
- SelenoH is localized to the nucleoli. Its expression is elevated during embryonic development. SelH has glutathione peroxidase activity. Its main function is to regulate the transcription of genes which are involved in de novo glutathione synthesis and phase II detoxification enzymes.
- SelenoV is only found in testes, so it may be involved in male reproduction. SelenoV has evolved by duplication of SelenoW but their N-terminal is different.
Selenoprotein I
SelenoI is found only in vertebrates and has a CDP-alcohol phosphatidyltransferase domain and contains seven transmembrane domains. The Sec residue is located in the COOH-terminal. The function of the Sec residue and SelenoI is not known.
15-kDa Selenoprotein
Sel15 is a thioredoxin-like fold ER-resident protein and contains an NH2-terminal signal peptide. It is expressed in prostate, liver kidney and testes. It is thought to have a role in protective mechanism of oxidative stress in the liver and cataract development and it prevents cancer development.
Selenoprotein M
SelenoM is a distant homolog of Sel15, thus it is also a thioredoxin-like fold ER-resident protein. But unlike Sel15, SelenoM is only found in the brain. It is involved in neuroprotection, preventing oxidative damage induced by H2O2. Moreover, overexpression of SelemoM in the brain has been shown to inhibit aggregation of β-amyloid protein, so it could prevent Alzheimer's disease.
Selenoprotein K and Selenoprotein S
SelenoK and SelenoS have different sequence, but they are studied together because they similar topology. Both SelenoK and SelenoS proteins contain a single transmembrane domain in the NH2-terminal, a glycine-rich segment and the Sec residues are in the COOH-terminal. They are found in ER membrane. Their exact function is unknown but it is suggested that both SelenoK and SelenoS are related to ER-associated degradation (ERAD) of misfolded proteins. In addition, they are also implicated in inflammation and immune response. It has been described several SelenoK/SelenoS homologous proteins which contain Cys residues instead of Sec, such as Romo1 (reactive oxygen species modulator 1).
Selenoprotein O
SelenoO is not-well characterized. It has the Sec residue close to COOH-terminal end of the protein. Its function is unknown.
Selenoprotein N
SelenoN is localized in ER as a transmembrane glycoprotein and it is mostly expressed during embryonic development. It is also found in adult skeletal muscle. SelenoN is involved in the maintenance of satellite cells and it plays a role in the regeneration of skeletal muscle tissue after injury or stress. It has been demonstrated that acts as a cofactor of RyR (ryanodine receptor), which is a calcium release channel that mediates the release of Ca2+ from the sarcoplasmic reticulum during muscle contraction. Thus, SelenoN also regulated intracellular calcium mobilization. Mutation in SelenoN gene are associated with early-onset muscle disorders (SEPN1-related myopathies).
Selenoprotein P
SelenoP is secreted into the plasma and contains almost 50% of the total Se in the plasma. SelenoP contains multiple Sec residues. This and the fact that is found in the plasma suggest that SelenoP might function as a Se supplier to peripheral tissues, mostly to brain and testes.
SBP2
It recognizes SECIS element in the 3'UTR and it recruits eEFsec.
eEFsec
Its main function is to recruit Sec-tRNA [Ser] Sec. It interacts with SBP2 bound to SECIS element and with rRNA, therefore it is crucial for Sec incorporation into selenoproteins.
PSTK
This protein specifically phosphorylates seryl-tRNA(Sec) to O-phosphoseryl-tRNA(Sec), an activated intermediate tRNA for selenocysteine biosynthesis.
SECp43
It binds to selenocysteyl-tRNA [Ser]Sec. It is required for methylation of the 2'-hydroxylribosyl moiety in the wobble position of the selenocysteyl-tRNA[Ser]Sec and enhances selenoprotein expression. It may also regulate shuttling of the SecS-selenocysteyl-tRNA[Ser]Sec complex between the nucleus and cytoplasm.
SecS family
SecS1 and SecS2. Sec synthase, a pyridoxal phosphate-dependent enzyme, esterifies the serine moiety of seryl-tRNA[Ser]Sec, forming an aminoacrylyl intermediate which is then exchanged with selenol.
SepHS
These two proteins synthesize the monoselenophosphate (activated form). SepHS 1 is involved in selenocysteine recycling, whereas SepHS 2 generates the active selenium donor in order to produce selenocysteine.
Information was extracted from Labunskyy et al., 2014 and Li et al., 2018 (for further information see References)