Selenium
Selenium is an essential micronutrient in many organisms and plays an important role in a wide variety of biological and physiological processes, like the immune response or mammalian development. For this reason, selenium deficiency is associated with pathophysiological conditions including heart disease, neuromuscular disorders, cancer or inflammation1.
Selenoproteins
It was always thought that there were only 20 different amino acids, but in recent discoveries scientists found out the twenty-first amino acid: Selenocysteine (Sel). The proteins containing selenocysteine are called Selenoproteins. Selenocysteine, a selenium-containing amino acid, presents oxidoreductase functions and it is encoded by the UGA codon. This codon normal function is stop the translation. In order to avoid this, organisms evolved the Sec insertion machinery to incorporate this amino acid at UGA codons. This process requires a cis-acting Sec insertion sequence (SECIS).
Selenoproteins are the ones mediating the biological effects of selenium in the organism. They are present in the three evolutionary life domains: eukarya, archaea and eubacteria, and they are also observed in viruses2.
Functions of selenoproteins
Selenoproteins have different biological functions. They participate in oxidoreduction processes, redox signalling, antioxidant defense, thyroid hormone metabolism, immune responses, protein folding and selenocystein synthesis. For this reason, they possess a strong correlation with human diseases like cancer, Keshan disease, virus infections, male infertility, abnormalities in immune responses and thyroid hormone function2.
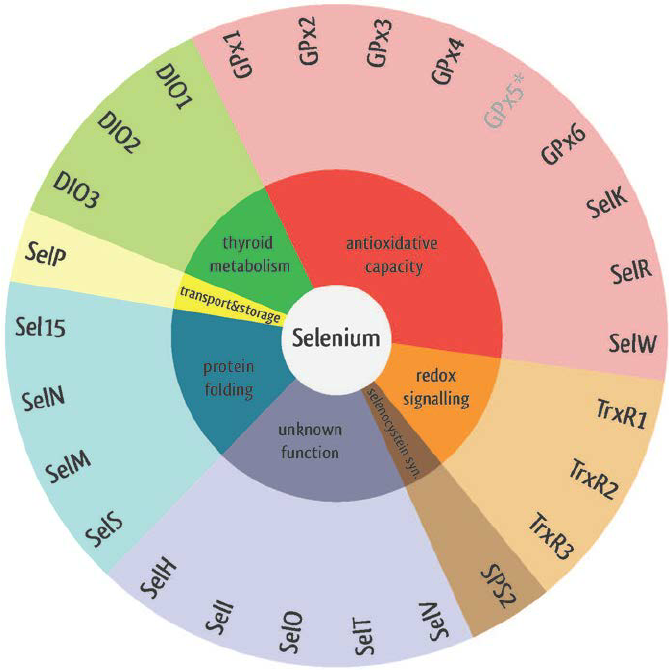
Image 1. Selenoproteins' functions2.
Selenoproteins biosynthesis
The pathway for its biosynthesis has been determined in recent years using a combination of comparative genomics, molecular, and structural approaches. The selenocysteine biosynthesis occurs on their own tRNA (tRNA[Ser]Sec). It was the longest tRNA ever sequenced (90-100 nucleotides). It presents unusual secondary structures and tertiary interactions3,4.
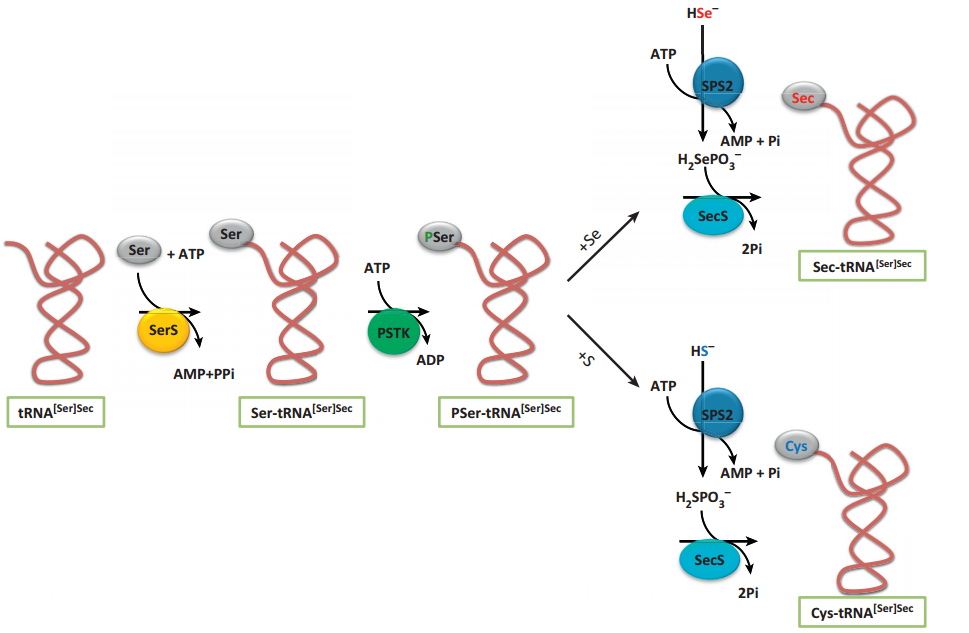
Image 2. Biosynthesis of selenoproteins2.
SECIS
Selenocysteine (Sec) is introduced into selenoproteins by a complex mechanism that requires trans-acting protein factors, Sec-tRNA[Ser]Sec and a cis-acting Sec insertion sequence (SECIS) element5. When a ribosome detect an UGA codon, which normally signals translation termination, Sec machinery interacts with the translation machinery to augment the coding potential of UGA codons and prevent premature termination6.
SECIS elements are the factors that dictate recoding of UGA as Sec and it is translated by Sec-tRNA[Ser]Sec. At least two trans-acting factors are required for efficient recoding of UGA as Sec in eukaryotes:
There are other additional SECIS-binding proteins:
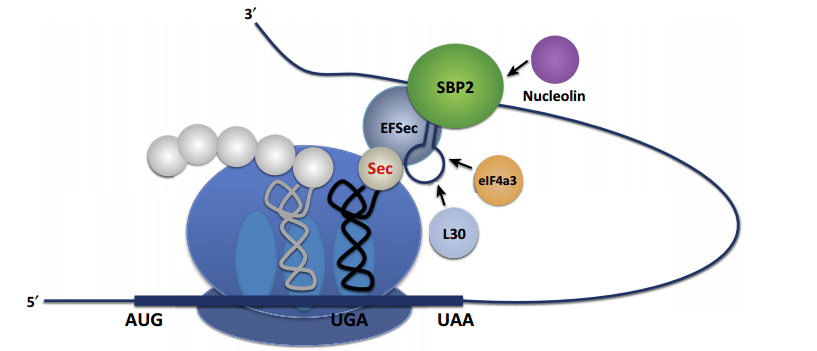
Image 3. Interaction of Sec machinery with a ribosome2.
Selenoproteins evolution
Selenoproteins are proteins that incorporate selenocysteine (Sec), an amino acid encoded by UGA. For its synthesis, it is necessary the enzyme Selenophosphate synthetase (SPS). This enzyme is conserved in all prokaryotic and eukaryotic genomes that encode selenoproteins. In many species, SPS is itself a selenoprotein, although there are homologs wit Cys instead of Sec. SPS proteins are conserved from bacteria to human with 30% identity approximately7.
Apparently, Sec can be substituted by cysteine without any, or almost any, variation on protein function. In eukaryotes, SPS is usually a selenoprotein, whereas in prokaryotes, it is common to find homologs with Cys aligned to Sec.
Also, Sec can be absent in a taxonomic group, but can be found in a similar lineage. It seems that there is certain selective pressure to maintain Sec, at least in vertebrates, based on cases of strong purifying selection across Sec sites that prevent mutations in Cys7.
SECIS (SelenoCysteine Insertion Sequence) elements are RNA structures responsible of changing the coding of UGA from a stop to a Sec. These elements have a stem-loop structure and are located in the 3’-untranslated regions (UTRs) of selenoprotein genes in eukaryotes and archaea, and immediately downstream of the UGA codon in the coding regions of bacteria. The human selenoproteome (the set of selenoproteins in an organism) is encoded in 25 genes, while in mice there are 24 selenoprotein genes.
It has been hypothesized that Sec is an ancestral trait, due to its presence in eukaryotes and prokaryotes. However, its continuity between domains is not certain, because it would require the translocation of SECIS elements and the radical alteration of its structure8.
Image 4. Phylogenetic tree, using PhyloT9.
Selenoproteins families
There are three characterized selenoprotein families: TrxRs, GPxs and DIOs. They have different enzymatic activities, but all require reductants to provide the electrons to make their catalytic redox cycle run.
The information about the function of each protein has been obtained from UniProt10.
Thioredoxin Reductases (TrxRs)
They are selenocysteines cointaining flavoenzymes that maintain thioredoxins in the reduced state using NADPH. Thioredoxins are small proteins that can catalyze redox reactions. The Sec residue is fundamental for the enzymatic activity and it is located in the C-terminal position. There are three thioredoxin reductases:
There are different isoforms. Isoform 1 has flutaredoxin and thioredoxin reductase activity and induces polymerization. Moreover, it regulates the intracellular redox environment. Isoform 4 enhances the transcriptional activity of estrogen receptors alpha and beta. However, isoform 5 only enhances the alpha activity but it also mediates cell death.
- Thioredoxin reductase 2 (TXNRD2)
It reduces proteins, has an impact on mitochondrial redox homeostasis and has an anti-oxidative stress activity.
- Thioredoxin reductase 3 (TXNRD3)
It has thioredoxin, glutaredoxin and glutathione reductase activities. Moreover, it promotes disulfide bond formation between GPX4 and sperm proteins. For this reason, it plays an important role in sperm maturation and in the formation of sperm components.
Glutation peroxidases (GPXs)
Glutation peroxidases can catalyze the reduction of organic hydroperoxides and hydrogen peroxide (H202) using glutathione, protects cells from oxidative damage and modulates the growth-factor mediated signal transduction, mitochondrial function and the redox-balance. There are six types of GPXs:
It protects the hemoglobin in erythrocytes from oxidative breakdown.
- GPX2
It protects against the toxicity of ingested organic hydroperoxides.
- GPX3
It protects cells and enzymes from oxidative damage and it catalizes the reduction of hydrogen, lipid and organic peroxides.
- GPX4
It is an essential antioxidant peroxidase that can reduce phospholipid hydroperoxide even if they are incorporated in lipoproteins or membranes. Moreover, it reduces fatty acid, cholesterol and thymine hydroperoxide; it prevents membrane lipid peroxidation and acccumulation of lipid reactive oxygen species. There is a selenocysteine in the active site that gives resistance to overodiation and prevents cells from ferroptosis.
- GPX5
It protecs cells and enzymes from oxidative damage, catalalize the reduction of hydrogen, lipid and organic hydroperoxide and constitutes a glutathione peroxidase-like protective system against peroxide damage in sperm membrane lipids.
- GPX6
It participates in the detoxification of hydrogen peroxide.
- GPX7
It protects esophageal epithelia from hydrogen peroxide-induced oxidative stress, suppresses acidic bile acid-induced reactive oxigen species (ROS) and protects from oxidative DNA damage and double-strand breaks.
- GPX8
It has glutathione oxidase activity in vitro and it catalyzes the oxidation of PDI in the presence of H2O2 and then it oxidizes PDI-catalyzed oxidation of glutathione to glutathione disulfide.
Iodothyronine Deiodinases (DIO)
Iodothyronine Deiodinases catalyzes the activation and inactivation of thyroid hormone by outer and inner ring deiodination. There are three DIOs:
It deiodinates T4 hormone (3,5,3,5-tetraiodothyronine) into T3 (3,5,3-triiodothyronine) and T3 into T2 (3,3-diiodothyronine). Moreover, it provides a source of plasma T3 by deiodination of T4 in peripheral tissues.
- DIO2
It deiodinates the hormone T4 into T3 and T3 into T2. It is also fundamental during the critical period of development, because it provides the brian with the necessary T3 levels.
- DIO3
It deiodinates T4 into RT3 (3,3,5-triiodothyronine) and T3 into T2. RT3 and T2 are inactive metabolites. Furthermore, it regulates circulating fetal thyroid hormone concentrations and the thyroid hormone inactivation during embryological development.
Other important selenoproteins
- MSRA
It has an important function as a repairing enzyme for proteins that have been inactivated by oxidation. Moreover, it catalyzes the reversible oxidation-reduction of methionine sulfoxide in proteins to methionine.
- MSRB1
It reduces methionine (R)-sulfoxide back to methionine. Methionine oxidation is a post-translational modification that takes place on specific residue. Acts as a regulator of actin assembly and plays an important role in innate immunity by reducing oxidized actin, leading to actin repolymerization in macrophages.
- MSRB2
It reduces methionine (R)-sulfoxide back to methionine and it plays a role in the preservation of mitochondrial integrity by decreasing the intracellular reactive oxygen species, contributing to cell survival and protein maintenance.
- MSRB3
It catalyzes the reduction of free and protein-bound methionine sulfoxide to methionine.
- Sel15
It participates in cell apoptosis and in the mediation of chemopreventive effects of selenium.
- SelH
It may be involved in a redox-related process.
- SelI
It catalyzes phosphatidylethanolamine biosynthesis from CDP-ethanolamine and it plays a central role in the formation and maintenance of vesicular membranes. It is involved in the formation of phosphatidylethanolamine via Kennedy pathway.
- SelK
It is required for Ca2+ flux in immune cells, as well as for palmitoylation and cell surface expression of CD36. It plays a role in T-cell proliferation and neutrophil migration. Moreover, it is involved in endoplasmic reticulum-associated degradation of soluble glycosylated proteins.
- SelM
It may function as a thiol-disulfide oxidoreductase that participates in disulfide bond formation.
- SelN
It plays an important role in cell protection against oxidative stress and in the regulation of redox-related calcium homeostasis. Moreover, it regulates the calcium level of the ER and acts as a modulator of ryanodine receptor (RyR) activity.
- SelO
It may be a redox-active mitochondrial selenoprotein which interacts with a redox target protein.
- SelP
It is responsible for some of the extracellular antioxidant defense properties of selenium and is involved in the transport of selenium. It is also possible that SelP supplies selenium to tissues such as brain and testis.
- SelS
It is involved in the degradation process of misfolded endoplasmic reticulum (ER) luminal proteins, transfering them from the ER to the cytosol, where they are destroyed by the proteosome.
- SelT
It has thioredoxin reductase-like oxidoreductase activity and protects dopaminergic neurons against oxidative stress and cell death. It is also involved in ADCYAP1/PACAP-induced calcium mobilization and neuroendocrine secretion and plays a role in fibroblast anchorage and redox regulation.
- SelU
Little is known about SelU function in Mus musculus. In chickens, SelU may regulate biological processes through its redox functions11. Moreover, its knockdown triggers autophagy through PI3K-Akt-mTOR pathway inhibition in rooster Sertoli cells12.
- SelW
It plays a role as a glutathione (GSH)-dependent antioxidant and is involved in a redox-related process. Moreover, it may play a role in the myopathies of selenium deficiency.
Selenoprotein machinery
- eEFsec
It is a translation factor necessary for the incorporation of selenocysteine into proteins and it replaces EF-Tu for the insertion of selenocysteine directed by the UGA codon.
- PSTK
It phosphorylates seryl-tRNA(Sec) to O-phosphoseryl-tRNA(Sec), an activated intermediate for selenocysteine biosynthesis.
- SecS
It converts O-phosphoseryl-tRNA(Sec) to selenocysteinyl-tRNA(Sec), incorporating a selenophosphate into the backbone of the amino acid, forming Sec-tRNA. It is the last enzime that takes part in the selenocysteine biosynthesis.
- SECP43
It is involved in the early steps of selenocysteine biosynthesis and Sec-tRNA charging to the later steps resulting in the cotranslational incorporation of selenocysteine into selenoproteins. Also, it is involved in the methylation of Sec-tRNA and it enhances efficiency of selenoproteins synthesis.
- SBP2
It binds to the SECIS element in the 3-UTR of some mRNAs encoding selenoproteins. This binding is stimulated by SELB.
- SPS1 & SPS2
Synthesizes selenophosphate from selenide and ATP.
Mus spicilegus

Image 5. Mus spicilegus
Taxonomy
The steppe mouse or mound-building mouse (Mus spicilegus) is a species of rodent in the family Muridae. This mouse is endemic in Europe. There are two recognised subspecies: M. s. spicilegus and M. s. adriaticus, an isolated sub-population on the Adriatic coast.
Description
The head-and-body length is between 70 and 80 mm and the tail is between 55 and 65 mm long. It is mostly a uniform grey, but there are some bicoloured populations. The tail is more slender compared to other related species. Moerover, it is very similar in appearance to the common house mouse (Mus musculus). The most significant difference is the mound-building proclivities of Mus spicilegus, however these are only apparent at certain times of the year.
Habitat
It lives in a variety of open habitats including natural steppe grasslands, pastures and cereal fields, orchards, open woodland, woodland edges and clearings. It avoids forests and human settlements.
Danger of extinction
According to the IUCN's Red List of Endangered Species, Mus spicilegus is a species that is not in danger of extinction, but has a minimal risk. This is because it is a common species and also lives in a very large habitat. However, it has a minimal risk due to the increase of extensive agriculture13.
For more information click here.