Conclusion
The aim of our study was to characterize and determine the selenoproteins and its machinery proteins in the recently sequenced specie Monopterus albus and to create a web page in html language in which we exposed all the parts of our study.
In order to analyze all the selenoproteins and machinery proteins in Monopterus albus, we used and automatized program, written in perl language. In this way we could obtain all the alignments between Danio rerio’s and Monopterus albus’ proteins and extract the predicted proteins. Furthermore, for those proteins that did not have a good alignment, we analyzed them manually using the exhaustive option of the exonerate.
Once we predicted all the corresponding proteins, we obtained the results for each one, which are indicated in the figure below:
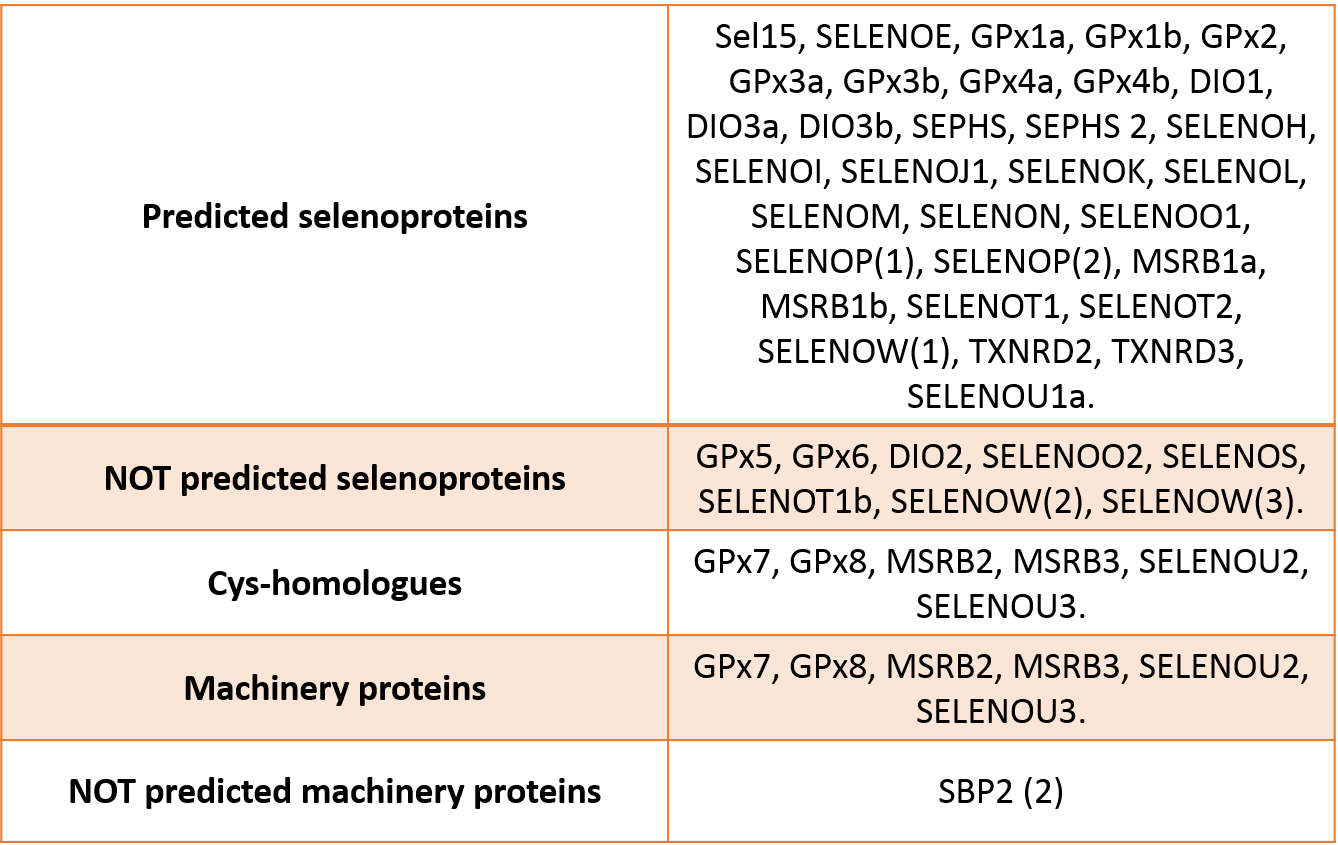
We could predict a total of 32 selenoproteins, 6 cys-homologues and 8 machinery proteins in Monopterus albus. On the other hand, 6 selenoproteins and 1 machinery protein from zebrafish could not be predicted: DIO2, SELENOO2, SELENOS, SELENOT1b, SELENOW(2), SELENOW(3) and SBP2 (2). Two other selenoproteins from Homo sapiens could not be predicted either, as expected: GPx5 and GPx6. Hence, we assumed that they are not present in this specie.
The results obtained in this study suggest that Monopterus albus’ genome is strongly conserved since almost all selenoproteins from Danio rerio could be predicted and characterized. Moreover, this results also suggest that there is good homology between Danio rerio’s and Monopterus albus’ genomes as expected because they both belong to Actinopterygii class of bony fishes.
The main limitation during this study was the faulty annotated genome of zebrafish in SelenoDB 2.0 as in some cases the proteins did not start with a Methionine and were not complete. Furthermore, the sequence of Monopterus albus genome contained lots of gaps and unread sequences. This affected our study in a couple of cases, where we predicted proteins containing “N”s instead of the defined nucleotides. However, the prediction of those proteins could be done due to the high homology and conservation observed in the regions where the sequence was properly obtained.
This study has characterized the selenoproteome of M. albus for the first time, contributing to the scientific society with its annotation.