INTRODUCTION
Selenium:
Selenium, which was originally considered as a toxin, is now defined as a fundamental element in human health due to its presence and contribution in several metabolic pathways, including antioxidant defence systems, thyroid hormone metabolism and immune function. It has been proved that a reduction of selenium concentration in blood is related to various chronic disorders, such as cancer and cardiovascular diseases. This element is added into proteins, as the majority of the metals, but it is also is added within a covalent bond in the 21st amino acid, in the genetic code as a selenocysteine (Sec) residue, which was first reported in 1978. In addition, the Sec residue structure is almost identical to the cysteine one, except that it has selenium instead of sulphur.

Selenoproteins:
There is an extensive number and variety of selenoproteins described due to their widespread in the nature. Moreover, concretely 45 selenoprotein families have been defined in vertebrates and 25 selenoproteins have been identified in humans up until now.
In order to achieve the incorporation of the selenocysteine residue, it is necessary to do a co-translational process, which applies the microsomal machinery for protein synthesis making it unique in certain steps. Curiously, Sec residue is the only amino acid synthesized directly on its transfer RNA (tRNA[Ser]Sec), and is not produced from a Cys but from a serine (Ser). On one hand, the selenocysteine residue is encoded by a TGA or an UGA triplet in DNA or RNA sequence, respectively. Moreover, it requires highly complex mechanisms for decoding the UGA because it could act as a selenocysteine or as a stop codon in non-selenoprotein genes. The UGA codon duality is circumvented by the presence of conserved cis- and trans- acting elements dedicated to the decoding of UGA as a Sec residue. On the other hand, the UGA triplet, as a selenocysteine codon, depends on the presence of some determined secondary mRNA structure. Thus, Sec residue is co-translationally added into the polypeptide chain in response to UGA codon, where a specific stem-loop structure, named SECIS (SEC Insertion Sequence Element), 60-nucleotide-length stem-loops that residue in the 3'UTR of eukaryotic selenoprotein mRNA, is located in the 3'UTR region of the gene. Hence, these characteristic structures have been first identified as stem-loops downstream of the UGA codon in bacteria, but in the eukarya and archaea it has been shown that the structure was form by remote stretches in the 3'UTR of the RNA. Furthermore, it has been described that this SECIS structure has to be present in all eukaryotic selenoprotein, and that the two codons preceding the UGA seem to modulate the SECIS efficiency. Then, a removal of SECIS structure, from the 3'UTR region of the gene, causes the stop of the selenoprotein synthesis.
It is important to point out that there is no available computational option to identify the function of the codon (UGA) as stop or selenocystein. Then, as a consequence of this key missing method to differentiate its function, several selenoproteins are misannotated, which leads to a loss of crucial information.

Biosynthesis of Selenoproteins:
As it has already been said, there are some conserved cis- and trans- mechanisms to decoding of UGA as a Sec residue. The main cis-acting elements for the synthesis of a selenoprotein are the SECIS element and the in-frame UGA codon. In contrast, the basic trans- acting elements are SPS1 (selenophosphate synthetase 1), SPS2 (selenophosphate synthetase 2), SecS (selenocysteine synthase), Pstk (O-phosphoryl-tRNASec kinase), eEFsec (eukaryotic elongation factor, selenocysteine-tRNA-specific), SBP2 (SECIS binding protein 2) and SeCys/tRNA[Ser]Sec (selenocysteyl-tRNA[Ser]Sec). In conclusion, all these elements and members are generally described as the selenoprotein translation machinery.

Phylogeny of vertebrate seleproteins:
There is still a lot of information missing regarding the genomes without a proper annotation for selenoproteins. Hopefully, the new technologies allow the researchers to rapidly progress in this direction.
The figure shows the evolution of the vertebrate selenoproteome. Understanding this figure will help us to predict different genetic events that have occurred during the evolution, such as duplications (indicated in green), Sec-to-Cys switches (indicated in blue), or losses of concrete selenoproteins (indicated in grey). The knowledge acquired through these selenoprotein analysis studies is very useful for studying the evolutionary processes.
The Nanorana parkeri is included in the frog lineage, and it is shown in the figure, Nanorana parkeri is phylogenetically close to primates regarding the selenoproteome. The number of selenoproteins, which is indicated on the right side of the figure, shows a higher value in all bony fishes in comparison to the rest of the vertebrates, in which Nanorana parkeri is included.
In our study, as we will focus on the frog lineage, it is important to highlight the Sec-to-Cys containing residue in defined selenoproteines, concretely SelU1, SelPb and Fep15.
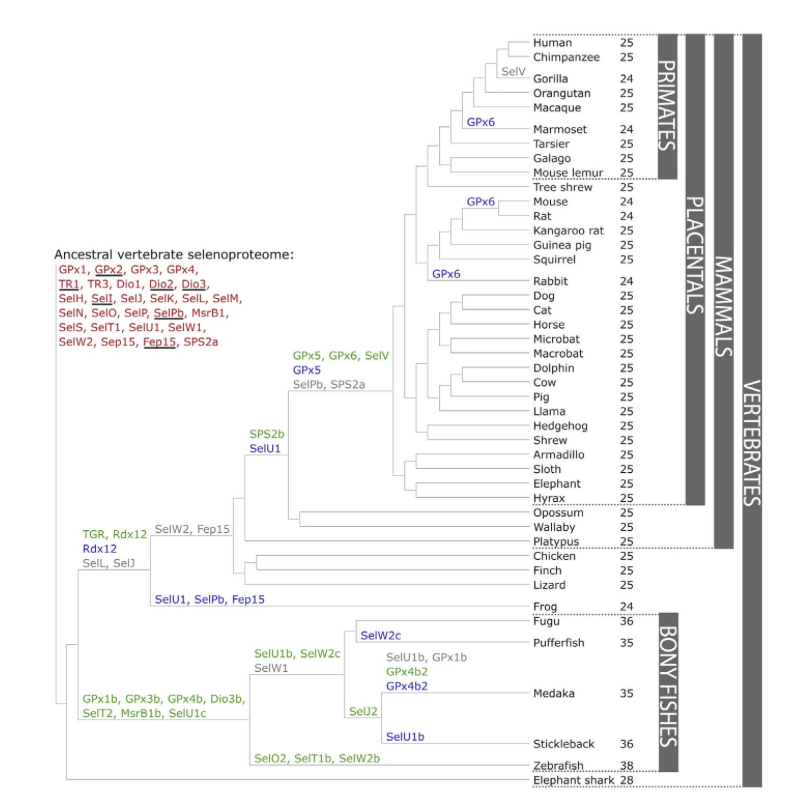
Figure 1. Evolution of the vertebrate selenoproteome. The ancestral vertebrate selenoproteome is indicated in red, and its changes across the investigated vertebrates are depicted along their phylogenetic tree. The ancestral selenoproteins found uniquely in vertebrates are underlined. The creation of a new selenoprotein (here always by duplication of an existing one) is indicated by its name in green. Loss is indicated in grey. Replacement of Sec with Cys is indicated in blue (apart from SelW2c in pufferfish, which is with arginine). Events of conversion of Cys to Sec were not found. On the right, the number of selenoproteins predicted in each species is shown.

Known Selenoproteins:
| DI | The iodothyronine deiodinases family (DI) is the general name for a family constituted of enzymes that catalyze the removal of iodine atoms from various thyroid hormones (Ths) in the thyroid gland and extrathyroidal tissues. As such, they are responsible for both the activation and inactivation of these compounds, and are thus important regulators of TH actions. |
| GPx | The glutathione peroxidase family (GPx) is the common name for a family of multiple isozymes that catalyze the reduction of H2O2 or organic hydroperoxides to water or corresponding alcohols using reduced glutathione (GSH) as an electron donor (H2O2 + 2GSH fi GS-SG + 2H2O). |
| Sel W ans Sel V | The selenoproteins W1 and W2 (SelW1 and SelW2) are members of the ancestral selenoproteome.Furthermore, SelV, which is the least conserved mammalian selenoprotein.The functions of SelV and SelW, both located in the cytosol, are not known, but SelV is expressed exclusively in testes, whereas SelW is expressed in a variety of organs. |
| Sel O | The SelO family is the common name for a family of ancestral selenoproteins found in all the vertebrates. These isozymes are localized to mitochondria and expressed in different tissues. |
| Sel P | The selenoprotein P (SelP) is an ancestral selenoprotein found in all the vertebrates. This protein is a secreted glycoprotein that contains most of the selenium in plasma. Although its function is unknown, it seems to have antioxidant properties. |
| Sel U | The selenoprotein U family (SelU family) is composed of three members (SelU1, SelU2 and SelU3).SelU function is unknown. |
| TR | The thioredoxin reductases family (TR) is a protein family composed by flavoproteins, which function as homodimers, actively involved in redox regulation of cellular processes due to their capacity to control the redox status of thioredoxins. |
| Sel R (Msr) | The Mrs enzymes (methionine sulfoxide reductases) are thiol-dependent enzymes, those function is to catalyze conversion of methionine sulfoxide to methionine. |
| Fep 15 and SelM | Fep15 was generated during the whole genome duplication occurred at the root of vertebrates, and it specially evolved by duplication of SelM. This last selenoprotein, SelM, which is a selenoprotein found in all vertebrates, shares 31% sequence identity with Sel15 in animals, followed by mutations that resulted in the loss of Cys in the region upstream of the Sec. |
| Sel H | The selenoprotein H (SelH) is an ancestral selenoprotein found in all the vertebrates and its function is not completely known. It is regarded as a nucleolar oxide-reductase with an antioxidant function, and it is also related with the gene regulation regarding de novo synthesis of glutation as it contains a DNA-binding-domain. |
| Sel I | The selenoprotein I (SelI) is an ancestral selenoprotein found in all the vertebrates. This protein is one of the more recently discovered selenoproteins. |
| Sel K | The selenoprotein K (SelK) is an ancestral selenoprotein found in all the vertebrates. This protein is a small selenoprotein that resides in the ER and in the plasma membrane. This protein has an unknown biological function. |
| Sel N | The selenoprotein N (SelN) is an ancestral selenoprotein found in all the vertebrates. This eukaryotic selenoprotein is located basically in the ER membrane. It has a high expression in fetal and growing muscular tissue, skeletal muscle, heart, lung and placenta. It has an important role in the diaphragm. |
| Sel Pb | SelPb) is an ancestral selenoprotein found in all the vertebrates, and it showed a partial conservation of intron structure with its closest homologs. SelPb function is unknown, but it seems to be responsible for some of the extracellular antioxidant defense properties of selenium. |
| Sel S | The selenoprotein S (SelS) is an ancestral selenoprotein found in all the vertebrates. This enzyme is located in the ER and the cell membrane.It has an important function in the UPR as it is part of the ERAD (Endoplasmic-reticulum-associated protein degradation) by retrotranslocating the misfolded proteins, and it provides protection against the ROS, concretely it is a thioredoxin-dependent reductase. |
| Sel T | The selenoprotein T (SelT) is an ancestral selenoprotein found in all the vertebrates. It is localized in the Golgi and the ER. It has an important role in the cell adhesion and enhances the expression of several oxidoreductase genes. |
| Sel 15 | The selenoprotein 15 (Sel15) protein is an ancestral selenoprotein found in all the vertebrates. It takes part of the Sep15 family and its specific function remains unknown. |

Known Selenoproteins Machinery:
| eEFsec | eEFsec includes a Sec amino-acid in a protein. |
| PTSK | the function and homology of this protein is conserved across archaea and eukaryotes that sinthetise selenoproteins, fact that suggests that it plays an important role in selenoprotein biosynthesis and/or regulation. |
| SBP2 | SBP2 promotes Sec incorporation by associating with SECIS elements and recruiting the eEFSec-selenocysteyl-tRNA[Ser]Sec complex to the ribosome. |
| SECp43 | SECp43 is required for the Sec-tRNA[Ser]Sec maturation, meaning that it is essencial for methylation of the 2'-hydroxylribosyl moiety in the tremble position of the selenocysteyl-tRNA[Ser]Sec and enhances selenoprotein expression. |
| SecS | SecS, a pyridoxal phosphate-dependent enzyme, esterifies the serine moiety of seryl-tRNA[Ser]Sec, forming an aminoacrylyl intermedite which is then exchanged with selenol. |
| SPS | SPS is unique among the components of the Sec biosynthesis machinery in that it is often a selenoprotein itself. This enzym catalyzes the synthesis of selenophosphate from selenide, ATP, and water. SPS is required for Sec synthesis, and it is conserved in all prokaryotic and eukaryotic genomes encoding selenoproteins. This enzyme is described itself as a selenoprotein in most of the species. |

Nanorana parkeri:
The High Himalaya frog Nanorana parkeri also known as Xizang Plateu frog, Parker's slow frog or mountain slow frog, lives at high altitude from 2850 to 5000m all over the Tibetan Plateau. Encompassing the southern and eastern Xizang, southern Qinghai in China, northeastern Kashmir, Himalayan Bhutan, Nepal and India.
It is able to survive when being exposed to extreme conditions, such as hypoxia, high UV radiation and notable changes in temperature during the day. Nevertheless, it is able to reproduce in suitable streams and marshes and shows a terrestrial adult lifestyle common in most anurans.
Finally, it is important to point out that Nanorana parkeri has a considerably larger genome than Xenopus tropicalis, as another well-known member of frog lineage, due to the number of transposable elements in both genomes. Moreover, members have a considerable conserved genome even having been diverged approximately 266Ma.

| Kingdom | |
| Phylum | |
| Class | |
| Order | |
| Family | |
| Subfamily | |
| Genus | |
| Species |
