Selenium and selenoproteins
Selenium is an essential micronutrient for mammals and humans that can be toxic when found in high concentrations in the body. Its role has been linked to its presence in selenoproteins, which are proteins that take part in many important physiologic processes such as redox reactions, redox signaling, antioxidant defense, thyroid hormone metabolism and immune response. That is the reason why a selenium deficiency has been associated to different diseases related to immune abnormalities, to cell damage (such as cancer) or to other diseases like Keshan Disease and Kashin-Beck Disease.[1, 2, 3]
A selenoprotein is a protein that contains a selenocysteine amino acid (Sec, U), the 21st amino acid. Sec is very similar to cysteine (Cys), but instead of sulfur it has a selenium atom, which is a much more reactive element. This amino acid is encoded by the TGA codon in DNA and decoded from UGA, which usually acts as a STOP codon. Thus, a complex translational mechanism is needed in order to incorporate Sec into the polypeptide chain. This mechanism implies the presence of a conserved loop-stem structure located in the 3'-UTR region that is called Sec insertion sequence (SECIS) element, as well as many other elements.[1, 2]
The tRNA needed to tranlsate UGA codon into a Sec amino acid has to suffer some modifications and interventions by other proteins. It is first aminoacylated with serine, then phosphorylated to form phosposeryl-tRNA[Ser]Sec by phosphoseryl-tRNA[Ser]Sec kinase (PSTK) and converted to selenocysteyl-tRNA[Ser]Sec by Sec synthase (SecS), the enzime that incorporates the selenium in a monoselenophosphate form. Selenophospate synthetases (SPS) are needed to convert the dietary selenium into this monoselenophospate form. Other additional proteins are needed in the process of Sec decoding, such as SECIS binding protein 2 (SBP2), the elongation factor EFSec, L30, Secp43 or Nucleolin. The three first participate in the binding of the tRNA to the poliA site, to the ribosome and to the SECIS element. [4]
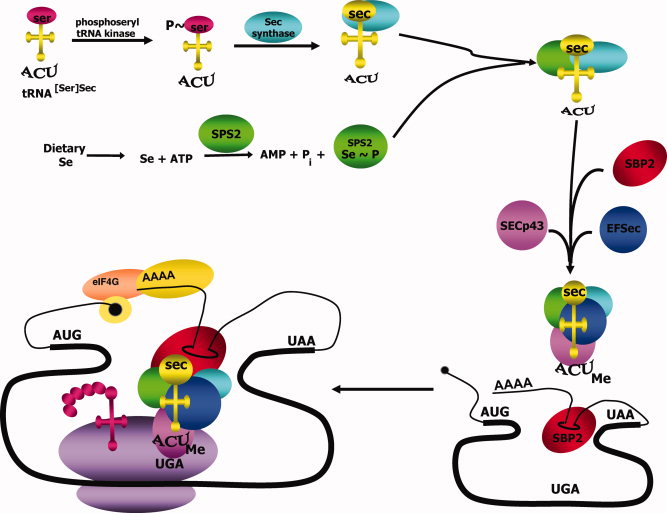
Figure 1: Selenocysteine biosynthesis and incorporation into selenoproteins.[4]
Selenoproteins are known to be present in the three domains: bacteria, archaea and eukaryote. Eukaryotes have a variable sets of selenoprotein that vary from zero selenoproteins in higher plants and fungi to more than 30 in some fishes and algae. Selenoproteomes are more similar to each other in closer related species, but at larger evolutionary distances some selenoproteins are lost in some phyla and a few evolve and duplicate in small sets of organisms. Species that have lost Sec during the evolution use Cys-containing homologs, although in general terms selenoproteins are more often conserved and evolved as Sec has a greater effectiveness in catalysis.[1]
The following figure shows a phylogeny of the vertebrates' selenoproteome. The ancestral proteome is indicated in red, the duplications in green, loss is indicated in grey and replacements of Sec to Cys in blue.[5]
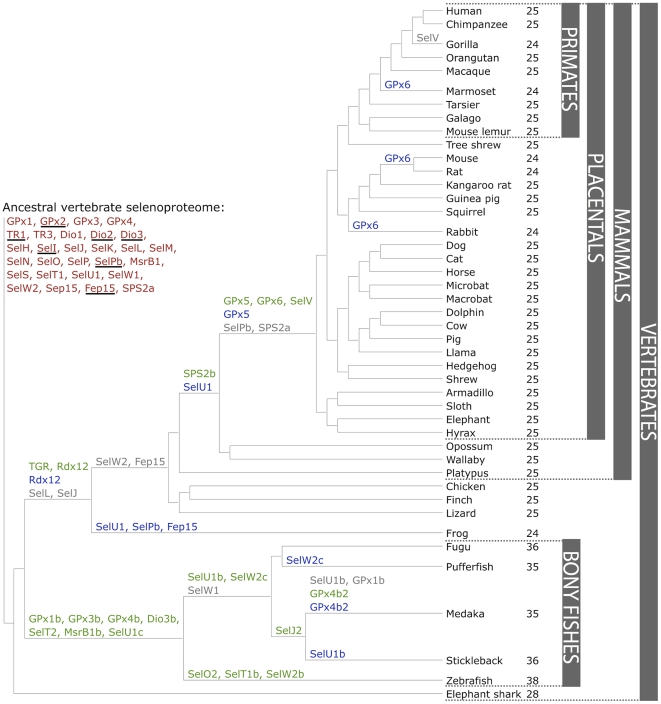
Figure 2: Evolution of the vertebrate selenoproteome.[5]
Selenoprotein families
To give our research some theoretical context, we want to describe a few details of the known physiological functions and evolutionary story of the proteins we have analyzed. It is important to know that it has been suggested previously that the ancestral vertebrate selenoproteome consists of 31 selenoproteins: Dio1-3, GPx1-4, SelH, SelI, SelJ, SelK, SelL, SelM, SelN, SelO, SelP, SelPb, MsrB1, SelS, SelT1, SelU1-3, SelV, SelW1, SelW2a, Sep15, SPS2, TR1, TR3 and TGR.[5]
Eukaryotic elongation factor family
A eukaryotic elongation factor (eEF) is a translation elongation factor necessary for the incorporation of Sec into proteins. It probably replaces EF-Tu, which is responsible for the selection and binding of an aminoacyl-tRNA to the acceptor site (A-site) of the ribosome, for the insertion of Sec directed by the UGA codon.[6] Thus, the Sec-specific eukaryotic elongation factor (eEFSec) is responsible for recruiting tRNA[Ser]Sec and, together with the SECIS binding protein 2 (SBP2), it inserts a Sec into the nascent protein chain in response to a UGA codon.[7]
Glutathione peroxidase family
Glutathione peroxidases (GPx) are the largest selenoprotein family in vertebrates. Mammals have 8 GPxs homologs, 5 of which are selenoproteins: GPx1-4, and GPx6, this last one only being present in humans.[5] These have an antioxidant function at different locations and cellular compartments and present a Sec in their catalytic centre:[8]
- - GPx1 ubiquitously in the cytosol and mitochondria.
- - GPx2 in the intestinal epithelium.
- - GPx3 in the plasma, so they work in the water phase.
- - GPx4 protects membranes from oxidative challenge.
- - GPx6 in the olfactory epithelium.
Furthermore, it seems like Cys-containing GPx7 and GPx8 evolved from a GPx4-like selenoprotein ancestor when the separation between mammals and fishes occured. On the other hand, GPx5 and GPx6 are the most recently evolved GPxs, originated from a tandem duplication of GPx3 with the appearance of placental mammals. Each of the mammalian Sec-containing GPxs genes are highly conserved.[5]
As far as their function, GPxs catalyze the reduction of H2O2 or organic hydroperoxides to water or alcohols with reduced glutathione. Some of them have a selenium-dependent glutathione peroxidase activity and present a Sec, which is the case of the proteins we will try to find in the genome of S. hypoxantha.[9]
Iodothyronine deiodinase family
The iodothyronine deiodinases (DIO) regulate the activation and inactivation of thyroid hormones. There are three DIO enzymes in the human genome, all of which contain Sec: DIO1, DIO2, DIO3. These proteins have a high homology between them and are found in all vertebrates. DIO2, unlike the others, is an ER-resident protein which activates the thyroid hormone by deiodination of the outer tyrosyl ring. DIO2 has a second in-frame UGA codon in its mRNA. In a cell culture, it was found that the second UGA could insert Sec when the first UGA codon was mutated, so it seems like the primary function of this second UGA is to serve as stop codon.[5]
DIOs regulate thyroid hormone maturation as they regulate the conversions between different forms of thyroid hormones: T3, T4 and reverse triiodothyronine (rT3). We find them expressed in different tissues:[10]
- - DIO1 is mainly found in liver, kidney, and thyroid.
- - DIO2 in brain, pituitary, thyroid, skeletal muscle, and brown adipose tissue.
- - DIO3 is abundant in placenta and uterus during pregnancy, as well as in cerebral cortex and skin.
Methionine sulfoxide reductase A family
Methionine sulfoxide reductase (MsrA) is the main peptide in the methionine sulfoxide reduction pathway. MsrA catalyzes thioredoxin-dependent methionine sulfoxide reduction.[11] This enzyme (as well as the MsrB or SelR) is present in all cell types, and have shown to be regulating life spans in mammals, insects, and yeast.[12]
Phosphoseryl-tRNA kinase family
Sec not only has its own nucleotide triplet, UGA, but also has its own tRNA. Biosynthesis of Sec occurs on its tRNA in both bacteria and mammals after the tRNA is initially aminoacylated with serine, this aminoacylation is done by seryl-tRNA synthetase. Phosphoseryl-tRNA kinase (PSTK) specifically phosphorylates the seryl moiety on seryl-tRNA[Ser]Sec. As a matter of fact, it has been found that tRNA[Ser]Sec is limiting for UGA translation under conditions of high selenoprotein mRNA.[13]
Proteins with homology to mammalian PSTK occur in Drosophila, Caenorhabditis elegans, Methanopyrus kandleri, and Methanococcus jannaschii, suggesting a conservation of its function across archaea and eukaryotes that synthesize selenoproteins and the absence of this function in bacteria, plants, and yeast. The fact that PSTK has been highly conserved in evolution suggests that it plays an important role in selenoprotein biosynthesis and/or regulation.[14]
SECIS binding protein family
Two trans-acting factors are required for efficient recoding of UGA as Sec in eukaryotes: SECIS binding protein 2 (SBP2) and Sec-specific translation elongation factor (eEFSec). SBP2 is stably associated with ribosomes and contains a distinct L7Ae RNA-binding domain that is known to bind SECIS elements with high affinity and specificity. Aside from binding to ribosomes and SECIS elements, SBP2 also interacts with eEFSec, which recruits Sec-tRNA[Ser]Secand facilitates incorporation of Sec into the nascent, growing polypeptide.[7]
Selenocysteine synthase family
Selenocysteine synthase (SecS) converts O-phospho-L-seryl-tRNA [Ser]Sec into selenocysteyl-tRNA [Ser]Sec using selenophosphate as the selenium donor compound.[15] SecSs, SelA in bacteria and SepSecS in archaea and eukaryotes, catalyze the terminal reaction of Sec synthesis during which either serine or phosphoserine is converted into Sec while being attached to tRNASec.[16]
Selenophosphate synthetase family
The selenophosphate synthetase family proteins are unique among the components of the Sec biosynthesis machinery as they may be a selenoprotein itself. They are conserved from bacteria to human with ~30% identity, and are found in all species known to encode selenoproteins. It catalyzes the synthesis of selenophosphate from selenide, ATP, and water, producing AMP and inorganic phosphate as products, being selenophosphate the selenium donor for the synthesis of Sec.[17]
The function of selenophosphate synthetase 2 (SPS2) is to generate the selenium donor compound (selenophosphate) necessary for Sec biosynthesis, and interestingly it is itself a selenoprotein. We find it in all vertebrates.In mammals, the SPS2 gene was initially a multiple exon gene (SPS2a), but was later replaced by a single exon copy (SPS2b). In non-mammalian vertebrates, only SPS2a is present.[5]
Unlike SPS2, SPS1 was found to be unable to produce selenophosphate in vitro. It has been suggested that SPS2 is required for de novo synthesis of selenophosphate, while SPS1 may have a possible role in Sec recycling through a selenium salvage system.[7]
Selenoprotein 15 and Selenoprotein M families
The 15-kDa selenoprotein (Sel15) and selenoprotein M (SelM) are thioredoxin-like fold ER-resident proteins that form a distinct selenoprotein family. These selenoproteins are only found in eukaryotes. The proteins have a thioredoxin-like domain and a surface accessible active site redox motif. They are involved in disulfide bond formation in the ER. In mammals, Sel15 expression is regulated by dietary Se, and either decreased or increased expression of this selenoprotein alters redox homeostasis. In this manner, Sel15 and SelM may have a physiological role as thiol-disulfide oxidoreductase and contribute to the quality control pathways of the endoplasmic reticulum.[18]
Sel15 was identified experimentally, and is thought to mediate the cancer prevention effect of dietary selenium and regulation of redox homeostasis in the ER. SelM is a distant homolog of Sel15, which was identified by bioinformatics approaches. Sel15 and SelM share 31% of sequence identity and demonstrate similar distribution, with homologs present from green algae to humans. Their expression was detected in a wide range of mammalian tissues, although their tissue expression patterns differ.[7]
Selenoprotein I family
Selenoprotein I (SelI) is a recently evolved selenoprotein, which is found only in vertebrates. It counts with the presence of a COOH-terminal extension-containing Sec residue, the function of which is currently not known.[7]
Selenoprotein K family and Selenoprotein S families
Selenoprotein K (SelK) belongs to the same protein family as selenoprotein S (SelS). Both of these proteins reside in the ER and contain a Sec. The exact function of SelK and SelS is still unknown, but in vitro studies have shown that they participate in anti-oxidant defense.[19]
Selenoprotein N family
It is thought that Selenoprotein N (SelN) participates in oxidative and calcium homeostasis, with a possible role in the regulation of the ryanodine receptor activity.[20] It plays an important role in cell protection against oxidative stress, and in the regulation of redox-related calcium homeostasis.[21]
Selenoprotein O family
No structural or biochemical characterization of Selenoprotein O (SelO) has been reported. Homologs of human SelO have been found in a wide variety of species including bacteria, yeast, animals, and plants. It contains a single Sec residue in the antepenultimate position at its the COOH-terminal end. It is important to note that the majority of SelO homologs contains a Cys residue in place of the Sec.[7]
Selenoprotein P family
Selenoprotein P (SelP) is an abundantly expressed secreted selenoprotein that accounts for almost 50% of the total selenium in plasma. The SelP protein family has recently evolved, and its homologs are found predominantly in vertebrates. The fact that SelP is secreted into the plasma and the presence of multiple Sec residues in its sequence suggests that this selenoprotein might function as a selenium supplier to peripheral tissues.[7]
Methionine-R-sufoxide reductase or Selenoprotein R family
Methionine sulphoxide reductases (Msr) catalyse the reduction of oxidized methionine (one of the most oxidation-sensitive amino acid) to methionine. These enzymes are divided into two classes, MsrA and MsrB, according to substrate specificity. In some bacteria these two enzymes are fused to form a single polypeptide (MsrAB), showin.[22]
MSRB1 is the only member of the family that is a selenoprotein, containing a Sec residue at its active site.[23] It is the main MsrB in mammals, which is primarily localized in the cytosol and nucleus. It has two homologs (MsrB2 and MsrB3) with a Cys residue in place of the Sec in the enzyme's active site, and different subcellular distributions but with similar catalytic efficiencies to that of MsrB.[7] In contrast to MsrA, which can catalyze the reduction of both free methionine-S-sulfoxide and its protein-based form, MsrB can reduce methionine-R-sulfoxide back to methionine only in proteins.[7]
Selenoproteins W, T, H, and V
Selenoproteins W (SelW), T (SelT), H (SelH), and V (SelV) belong to the Rdx family of selenoproteins. Based on the presence of the thioredoxin fold and the Cys-x-x-Sec motif, it was proposed that the Rdx family proteins are thiol-based oxidoreductases, but the exact function of these proteins remains unknown.
SelW is localized in the cytosol and is expressed at high levels in muscles and brain. It belongs to the stress-related group of selenoproteins as its expression is highly regulated by the availability of selenium in the diet.
SelT is predominantly located in the ER and Golgi. Knockdown of SelT in mouse fibroblasts leads to decreased expression of extracellular matrix genes involved in cell structure organization and alters cell adhesion properties. Its loss results in the upregulation of several oxidoreductase genes, including another member of the Rdx family, SelW. It is also thought to have a role in the regulation of Ca2+ homeostasis and neuroendocrine function, and is known to bee implicated in the regulation of pancreatic β-cell function and glucose homeostasis.
SelH is a mammalian protein with no homology to functionally characterized proteins. However, certain similarities to other selenoproteins can be seen, particularly a conserved Cys-X-X-Sec motif in which Cys and Sec are separated by two other amino acids (X). This feature is also present in several other mammalian selenoproteins like SelW, SelT, SelM, and SelV.[24] It resides mainly in the nucleoli, and has a role in redox regulation, being able to transactivate the expression of a couple of genes against oxidative stress. Thus, SelH appears to be a sensor of oxidative stress in the nucleus, where it may play a dual role in redox maintenance as an antioxidant and in regulation of gene expression as a transactivator.[25]
SelV is a recently evolved selenoprotein, most likely by duplication from SelW, and is found only in placental mammals. However, it was specifically lost in some organisms including gorillas. It is a larger than SelW due to the presence of an additional NH2-terminal domain, the function of which remains unknown. It has only been found to be expressed in testes, and thus may be involved in male reproduction, but its specific function is not known.[7]
Selenoprotein U family
Selenoprotein U's (SelU) homologs in non-fish species, contain Cys instead of Sec. And, its specific functions are still unknown. Chicken SelU is believed to be an important member of the avian selenoprotein family. The Sec is located close to a conserved Cys such that the two residues form a motif that resembles the UxxC/CxxC motif present in various thiol-dependent redox proteins. Its 3D structure suggests that chicken SelU might have the same functions as thiol-dependent redox proteins due to the conserved motifs. It is abundantly expressed in testes and brain.[26]
Thioredoxin reductase family
Thioredoxin reductases (TRs) control the redox state of thioredoxins, key proteins involved in redox regulation of cellular processes. Mammals have three TR isozymes: cytosolic and nuclear TR1, mitochondrial TR3, and TR2 or TGR. TRs are oxidoreductases that, together with thioredoxin (Trx), comprise the major disulfide reduction system of the cell. In mammalian cells, there are three TR isozymes, all of which are Sec-containing proteins. These proteins contain a Sec residue in the COOH-terminal penultimate position. Both TR1 and TR3 are present in all vertebrates. TGR's physiological function remains unknown, it is expressed at high levels in testis after puberty, and it thought to be involved in the formation/isomerization of disulfide bonds during sperm maturation.[7]
tRNA Sec 1 associated protein 1 family
SECp43 participates in the biosynthesis of selenoproteins together with the soluble liver antigen (SLA) through interaction with tRNA[Ser]Sec in a multiprotein complex. Recent studies suggest that SECp43 may also promote shuttling of SLA and Sec tRNA[Ser]Sec between different cellular compartments.[27]
Sporophila hypoxantha
Sporophila hypoxantha, known as tawny-bellied seedeater, is a species of bird of the genus Sporophila in the family Thraupidae, that lives in the centre and eastern side of South America.
The tawny-bellied seedeater can be found in the central territories of Brazil and practically the whole of the Bolivian territory, Paraguay, western Uruguay and the nordic provinces of Argentina.[28] It lives in the savannahs and humid pastures.[29]
Thanks to the large extension of territory that it occupies, it is in minimum risk of extinction. It cannot be considered neither threatened nor near-threatened regarding his conservation.[29]
As the rest of species of its genus, the tawny-bellied seedeater has a seed-based diet, which, in fact, gives name to the genus' common name: Seedeater.[30]
As far as behaviour, a characteristic of this species is that they have a very territorial behaviour. On the other hand, its song has been studied quite extensively, and has been described as melodious with sweet whistles, pleasant to the ear. In the species, we can find five regiolects (variants of songs found in subpopulations of a species with a big extension and that is present in all the individuals of this big rank), that correspond to different patterns of use of habitat, but that appeared independently of the presence of the migratory behaviour.[31]
For more information you can visit Wikipedia.