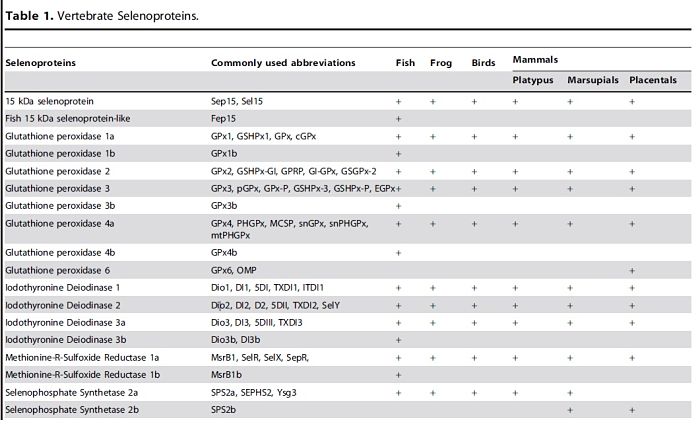
Eukaryotes have a size of the selenoproteome significantly different. The highest content of selenoproteins is observed in aquatic organisms, whether they are animals (e.g., fish) or plants (e.g., algae). The selenoprotein evolution is influenced by the environment in which organisms live and the availability of Se. On one hand, the availability of constant supplies of Se in the sea water could have made it possible to increase the use of this element for various oxidoreductases. On the other hand, selenoproteins are more susceptible to oxidative damage, so in higher oxygen levels environments, selenoproteins may be replaced by cysteine homologs.[3,10]
Although fish selenoproteomes are larger than those of mammals, the same core selenoprotein families are found in mammals and fish. In addition, fish have several selenoproteins (Fep15, SelJ, SelL) that are missing in mammals, as well as several Sec-containing copies of Selenoproteins T, U and W, and two forms of SelP.[3,10]
Several selenoproteins genes are found duplicated in bony fishes probably owing to the whole genome duplication in the early evolution of ray-finned fishes. In zebrafish there are only additional copies of SelO, SelT1 and SelW2, named respectively SelO2, SelT1b, and SelW2b.[3,10]
Gpx – Gluthatione peroxidase
Glutathione peroxidase is one of the largest and best-known selenoprotein family. The members of this group catalyze a reacction which forms gluthathione disulfide from two molecules of reduced gluthatione. Thus, they function in the detoxification of hydrogen peroxide.
Four Gpx have been described in fishes: classical GPx1, gastrointestinal GPx2, plasma GPx3 and phospholipid hydroperoxide GPx4. Each type of GPx has a physiological localization and substrate specificity, so collectively they provide a wide spectrum of antioxidant protection.[11]
Dio – Iodothyronine deiodinase
This selenoprotein family consists in three enzyms (types 1, 2 and 3) which are anchored on the membrane and share substantial sequence homology and catalytic properties.[11]
Iodothyronine deiodinase enzymes control activation and inactivation of thyroid hormones. The types DIO1 and DIO2 catalyze the 5'5-mono-deiodination of the pro-hormone thyroxin (T4) to the active thyroid hormone 3,3'5-triiodothyronine (T3). And the type DIO3 degrades both hormones by inner ring deiodination (IRD). Thus, thyroid hormone metabolism is dependent upon the combined actions of the three deiodinases.[11]
Sep15/Sel15 – 15 kDa Selenoprotein
The specific function of this selenoprotein is not known yet. Sel15 levels differentially respond to selenium supplementation. Studies in mouse suggest that this selenoprotein may have redox function and may be involved in the quality control of protein folding.[11]
Fp15 – Fish 15-kDa selenoprotein-like
The function of this protein is not clarified yet. But it is known that Fep15 is distantly related to members of the 15 kDa selenoprotein (Sep15) family. Fep15 is absent in mammals, can be detected only in fish and is present in these organisms only in the selenoprotein form. In contrast with other members of the Sep15 family, which contain a putative active site composed of Sec and cysteine, Fep15 has only Sec.[12]
MsrB – Methionine sulfoxide reductase B
It’s also named Selenoprotein R (SelR). This selenoprotein is part of the methionine sulfoxide reductase (Msr) family, which protect cells by repairing oxidatively damaged methionine residues in proteins. MsrB is widely expressed in a variety of tissues and its distribution is both perinuclear and cytosolic.[9]
SPS/SelD – Selenophosphate synthetase
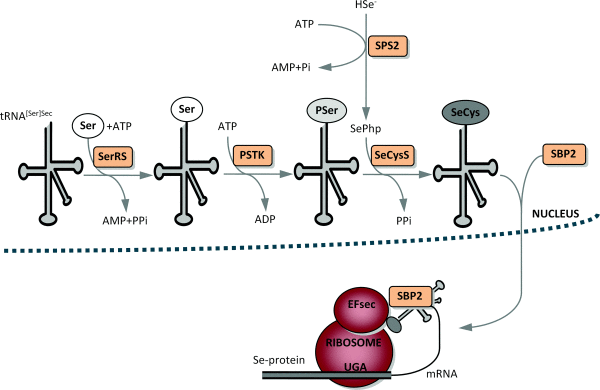
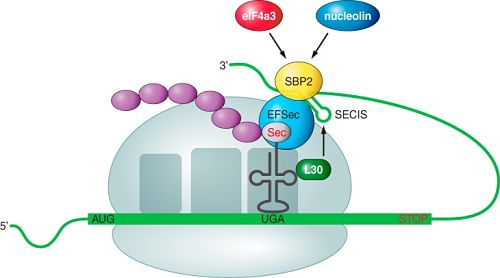
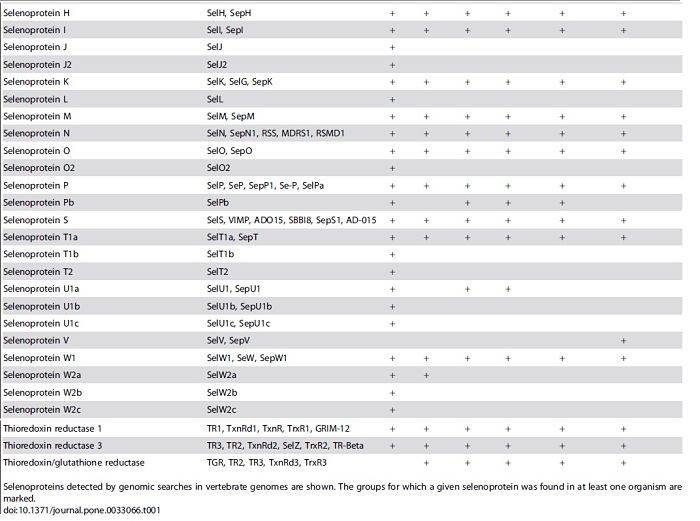
This selenoprotein is required for Sec synthesis and it’s conserved in all prokaryotic and eukaryotic genomes encoding selenoproteins. SPS is itself a selenoprotein in many species, although it’s common to find functionally equivalent homologs that replace the Sec site with cysteine (Cys). [8]
SPS2 is used as a marker for Sec utilization in eukaryotes This image shows SPS genes and predicted selenoproteins found in a representative set of eukaryotic genomes. The presence of SPS2 genes (defined as those with Sec or Cys) correlates perfectly with the presence of selenoproteins.[8]
Phylogenetic profile of SPS genes and approximate selenoproteome size of eukaryotes. (Mariotti M., et al; 2015)
SepH/SelH – Selenoprotein H
Selenoprotein H is an antioxidant protein strongly expressed in the developing CNS of vertebrates and is essential for embryonic development in zebrafish, since SelH induce MeHg downregulation in zebrafish and, consequently reduce Me HG toxicity.[13]
SepI/SelI – Selenoprotein I
It’s localized in the membrane (as SelK, S, T andN) and forms selenysulfide bonds leading to the formation and stabilization of protein complexes required for protein trafficking. Its particular function is not known. There is no relevant information about the expression in different tissues.[10]
SepJ/SelJ – Selenoprotein J
In contrast to known selenoproteins, the expression of SelJ appears to be restricted to actinopterygian fishes and sea urchin, with Cys homologues only found in cnidarians. SelJ shows significant similarity to the jellyfish J1-crystallins and with them constitutes a distinct subfamily within the large family of ADP-ribosylation enzymes. This protein is expressed preferably in the eye lens in early stages of zebrafish development. [14]
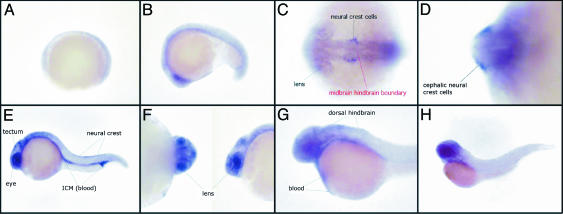
This image shows the expression pattern of the SelJ gene during development in zebrafish embryos. Shown are a gastrula (A), an embryo at middle somitogenesis (B), dorsoventral (C), or transversal (D) views of the 20-somite stage, a whole embryo 24 h after fertilization (E) and a closer view of the eyes (F), a whole embryo 36 h after fertilization (G), and an embryo 48 h after fertilization (H). All views are lateral, except C and D.(C) Territories in which SelJ is expressed are indicated in black, and the position of the midbrain–hindbrain boundary is shown in red.[14]
SepK/SelK – Selenoprotein K
Selenoprotein K is a small (16kDa) protein localized in the endoplasmatic reticulum membrane and some evidences suggest that it’s involved in endoplasmatic reticulum associated-degradation (ERAD) of soluble glycosylated proteins and plays a role in the protection of cells from ER stressed-induced apoptosis and from oxidative stress. Finally, it’s described that SepK is required for Ca+2 flux in immune cells.[10]
SepL/SelL – Selenoprotein L
This selenoprotein contains two Sec separated by two other residues, forming a UxxU motif. SelL proteins show an unusual occurrence, being present in diverse aquatic organisms, including fish, invertebrates, and marine bacteria and they aren’t present in mammals. Some evidences suggest that SelL has a redox function.[15]
SepM/SelM – Selenoprotein M
This selenoprotein is 15kDa and shares 31% sequence identity with Sel15. Both of them are localized at ER. Different studies suggest it as thiol-disulfide oxidoreductase, and also play a role in ER protein-folding.[10]
SepN/SelN – Selenoprotein N
It’s a transmembrane protein localized at ER membrane. In humans it’s seen that mutations in the gene coding for SelN cause different forms of congenital muscular dystrophy. These muscular diseases are characterized by early onset of hypotonia which predominantly affect in axial muscles. Zebrafish SelN is highly homologous to its human counterpart and amino acids corresponding to the mutated positions in human muscle diseases are conserved in the zebrafish protein. The sepn1 gene is highly expressed in the somites and notochord during early development. Inhibition of the sepn1 gene causes muscle architecture disorganization and greatly reduced motility. Therefore, SelN has an important role for muscle organization during early development. [16]
SepO/SelO – Selenoprotein O
Using bioinformatics tools, it’s predicted that SelO protein adopts a three-dimensional fold similar to protein kinases. Furthermore, SelO kinases might have retained catalytic phosphotransferase activity. Lastly, the role of the selenocysteine residue in its Cys-X-X-Sec motive suggest the possibility of an oxidoreductase-regulated kinase function for SelO. [17]
SepS/SelS – Selenoprotein S
This is a transmembrane protein located in the ER and plasma membranes and it’s widely expressed in a variety of tissues. SelS is involved in the degradation process of misfolded endoplasmic reticulum (ER) luminal proteins. It also participates in the transfer of misfolded proteins from the ER to the cytosol, where they are destroyed by the proteasome in a ubiquitin-dependent manner. Finally it’s been suggested to protect cells from oxidative damage and ER stress-induced apoptosis. Expression of SelS has been shown to be modulated by glucose metabolism and ER stress.[10]
SepT/SelT – Selenoprotein T
Selenoprotein T (SelT) is a member of a subfamily of selenoproteins (also including SelW, SelH and SelV) that share sequence similarity containing a thioredoxin-like fold and a conserved Cys-X-X-Sec motif. The expression of SelT is proposed to be similar to those selenoproteins involved in stress-related phenomena. SelT has shown to have a biological role in calcium mobilization.[10]
SepP/SelP – Selenoprotein P
In the eukaryotic kingdom, selenoprotein P (SelP) is the selenoprotein family that contains the most Sec residues. There are 10 Sec residues in human SelP and up to 17 in that of zebrafish.[18]
The function of SelP is not known. Several recent reports suggested that this protein may have antioxidant properties. It can attach to epithelial cells and protect against diquat-induced oxidative damage (Isolated SelP was reported to have phospholipid hydroperoxide glutathione peroxidase activity. In addition, SelP, owing to its high Se content, was implicated in the selenium delivery systems, such as selenium intercellular transport and storage.[18]
SepU/SelU – Selenoprotein U
Selenoprotein U (SelU) was firstly found in fish and also reported in birds and unicellular eukaryotes. In high mammalian species, such as humans and mice, all SelU proteins exist in Cys form. Its function is not known.[10]
SepW/SelW – Selenoprotein W
This selenoprotein interacts with glutathione and evidence suggest that it plays a role as a glutathione (GSH)-dependent antioxidant. It may be involved in a redox-related process and protects the developing of myoblasts from oxidative stress. It is also involved in the myopathies of selenium deficiency.[10]
TR – Thioredoxin reductase
Thioredoxin reductase (TR) enzymes are oxidoreductases that use NADPH to catalyze the reduction of oxidized thioredoxin (Trx). Trx is in turn used by several cellular enzymes as a cofactor in dithiol-disulfide exchange reactions and this is a major mechanism by which a reduced environment is maintained within cells, particularly serving to maintain reduced cysteine groups. There are three mammalian TRs: cytoplasmic/nuclear TR1, mitochondrial TR2, and testes-specific thioredoxin-glutathione reductase TR3.[10]