Iron Response ElementS
![]() STATE OF ART
STATE OF ART
![]()
![]() IRPs
IRPs
IRPs proteins, (IRP-1 and IRP-2) are aconitase superfamily homologues. Surprisingly as it happened with IREs containg mRNAs, its sequence identity are each more highly conserved between species than for IRP-1 and IRP-2 in the same species (90% vs 60-70%).
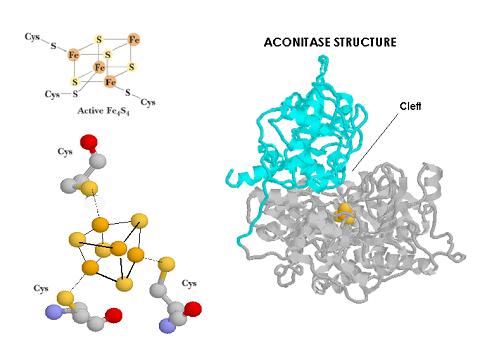
lthough IRP-1 (98KD) was the first IRP identified its nature has not been well characterized yet. When peptides from IRP1 are compared with those from c-aconitase, an identity higher of 98% is obtained. Complementary studies have shown by crystalography that aconitase presents in the cleft between the two subunits a Fe-S cluster. A Fe4S4 sulfur cluster that can convert in vitro IRP-1 into an aconitase. Assuming that the IRP-specific insertions are only in the surface loops that do not affect folding, the IRE binding site has to be close to or the same as the binding cleft of the Fe-S cluster. That is why it has been suggested that IRP-1 cycles between the RNA binding form and cytoplasmic aconitase up to the 4Fe-4S cluster presence or absence. It would be then more suitable to talk about a bifunctional protein. Iron up to the cellular status can drive IRP-1 regulation by building or not an Fe-S cluster on the cleft protein which blocks mRNA binding activity.
Although IRP-2 (105KD) is quite similar to IRP-1 (61% of sequence homology) does not forma an Fe-S cluster. The difference in sequence resides in de 73 amino acid insertion unique to IRP-2 near the amino terminus. IRP-2 in comparison with IRP-1, is not structurally regulated by iron. What iron makes in this case is decrease the cytoplasmic pool of IRP-2 through protein oxidation, followed by its ubiquitination and proteasomal degradation.
The redox state of cystein residues in both IRPs and complexation with the Fe-S cluster in IRP1 can influcence RNA binding and provide a potential site for regultaion by oxygen/hypoxia, nitric oxide, ascorbate, hydrogen peroxide and many oxyradicals (note all this stimules bring us to other proteins that as told before present in their mRNAs IREs structures too).