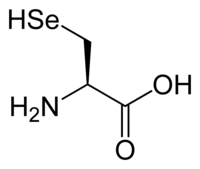
Plasmodium gallinaceum is a protozoan parasite that causes Malaria in domestic fowl, especially hens, when it infects them. The parasite is transmitted by the mosquito vector
Aedes aegypti.
P. gallinaceum was first described by Émile Brump in 1395 and isolated from a domestic chicken in Sri Lanka. It is an endemic in several African and Asian countries where it causes low mortality in the hostsafter the primary infection. However the avian malaria caused by
P. gallinaceum has a mortaility rate of 90% in birds from European countries.
The life cycle of
P. gallinaceum is very complex and resembles the other
Plasmodia species cycles substantially except in the first stages of the infectious cycle in birds as seen below.

Mosquito Aedes Aegypti, intermediate host of P. gallinaceum. Bites domestic fowl and can cause avian malaria.
Life cycle of Plasmodium gallinaceum
Development stages in the mosquito, sporogonic cycle
Exflagellation
The cycle begins when the mosquito bites an infected bird and ingests the
P. gallinaceum gametocytes. Once in the intestinal tract of the mosquito, the gametocytes differentiate to become gametes. Eight haploid gametes are produced from a single gametocyte during a process known as exflagellation. Then the gametes fusion takes place to form a zygote which finally becomes a ookinete.
In order to infect efficiently, the paraiste must cross two physical barriers: the peritrophic matrix and the intestinal epithelium.
Crossing the peritrophic matrix.
The peritrophic matrix is a chitin-containing acellular sheath that separates the food bolus from the midgut epithelium of the insect and it is formed in response to the typical intestinal distention as a consequence of food ingestion. To cross such barrier, the ookinete secrete chitinases which partially degrade the chitin matrix.
Invasion of the intestinal epithelium.
The ookinetes which cross the peritrophic matrix must invade the epithelium cells. To do so they adhere to the microvilli surface by an unknown specific molecular recognition process.
Once in the cell, the ookinete forms an oocyst on the outer wall of the midgut. The nucleus of the oocyst divides into thousands of sporozoites which migrate to the mosquito blood stream.
Invasion of the salivary gland.
From all the tissues in the organism, the sporozoits invade specifically the salivary gland which suggests a high molecular specificity for the salivary gland cells. Having reached the salivary gland, the sporozoites invade the cells in the gland. Then they are released to the secretory cavity. From now on, every time that the mosquito bites a bird a reduced number of sporozoits are transmitted to the bird blood consequently infecting it.
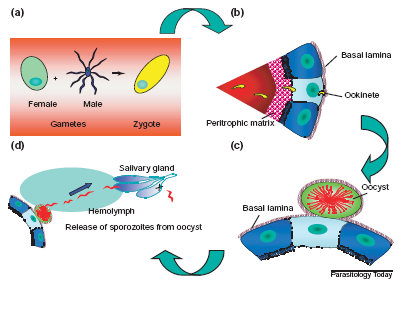
P. gallinaceum life cycle in the mosquito stages.a) Fertilisation and zygote formation. b) Ookinetes cross the peritrophic matrix, invade the intestinal epithelium,
c) cross the lamina basalis and become oocysts.d) Finally, the oocyst divides into eight sporozoits which migrate towards the salivary glands to be transmitted to the bird by the mosquito bite.
Bird infection
Exoerythrocytic cycle
Unlike the rest of Plasmodium species, P. gallinaceum does not invade hepatocytes, it invades the macrophages present in the zone of the mosquito bite. The sporozoites develop into cryptozoites in the macrophages, the first exoerythrocytic forms of the parasite present in the host. In the following stages, cryptozoites develop into merozoits, which are present in all the malarial infections by Plasmodium. From this stage on, the cycle follows as in any other Plasmodium species.
Erythrocytic cycle
The merozoits start the invasion process of the erythrocytes during which they develop into trophozoites and schizonts. These schizonts finally become schizogonies, and having reached this stage, the erythrocytes die and release the schizogonies and all the earlier forms in development. In such conditions, the merozoits can invade other erytrhocytes worsening the infection.
Gametogony stage
During this stage, the schizogonies develop into gametocytes, the asexual forms. The cycle is completed when a mosquito takes a considerable amount of gametocytes after biting an infected bird.
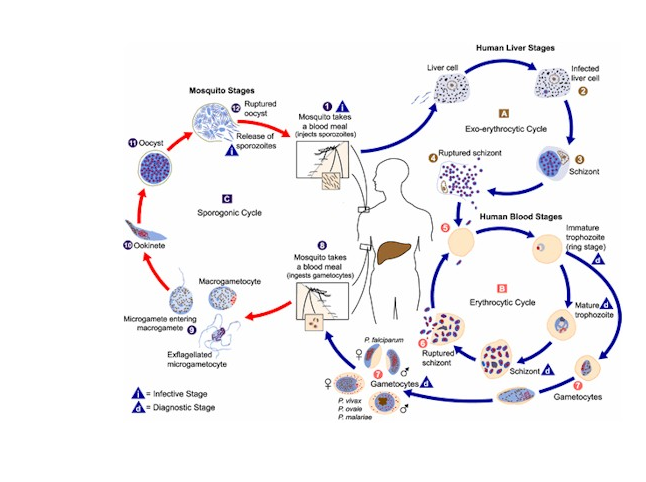
Life cycle of several Plasmodium species. The cycle can be divided in three cycles: a) The exoerythrocytic cycle: the parasite infects the hepatocyte and
develops. In the infection by P. gallinaceum the cells infected are the machrophages in the zone of the bite but the posterior development process takes place as well.
b) The erythrocytic cycle: after the development process of the parasite, the erythrocyte bursts releasing merozoits or schizogonies. The released schizogonies go
under a sexual differentiation process to become gametocytes. c) Sporogonic cycle: previously described, it includes the development stages of the parasite in the
mosquito.
Back to top